
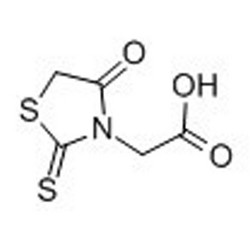
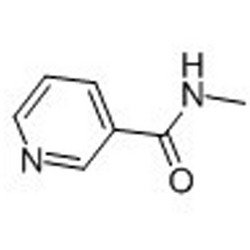
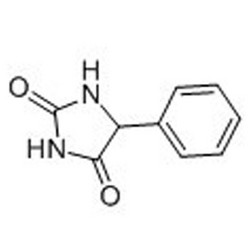
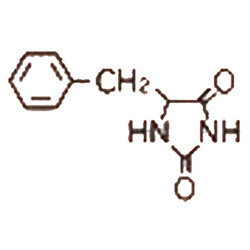
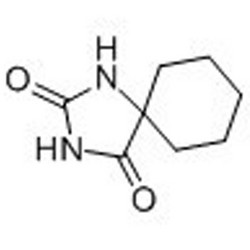
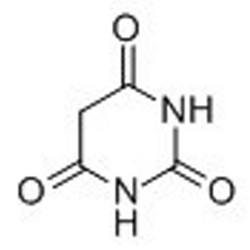
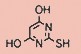
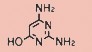
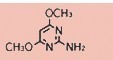
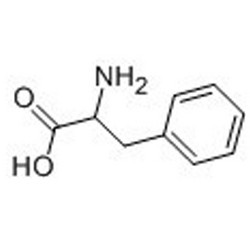
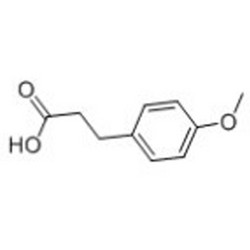
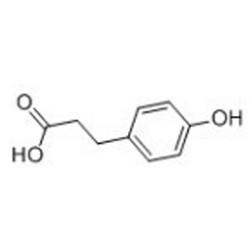
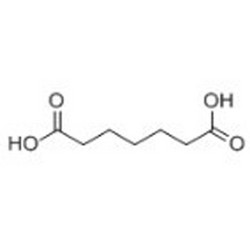
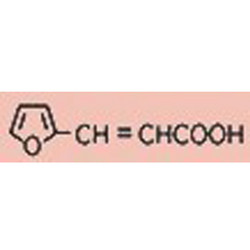
Tateyama Kasei was established in 1953 as a manufacturer of pharmaceutical bulk products and intermediates. In addition to our performance achievements, we have also reinforced with regard to overall quality control and quality assurance represented by GMP certification and ISO registration. Accepted FDA audit, we receive the high reputation and trust from our customers.




智藥研習社官方微信

制藥在線(xiàn)官方微信
 2006-2025 上海博華國際展覽有限公司版權所有(保留一切權利)
滬ICP備05034851號-57
2006-2025 上海博華國際展覽有限公司版權所有(保留一切權利)
滬ICP備05034851號-57